Infections are responsible for significant morbidity and mortality in haematological patients, in particular during chemotherapy-induced neutropenia and immediate initiation of empirical antibiotherapy is mandatory for patients exhibiting febrile neutropenia (1,2). The choice of antibacterial therapy should be adjusted for each patient and each centre. This standard has been applied since the 1970s following publication by Pizzo and Schimpff which demonstrated its benefits on infectious related mortality (3,4). Subsequent studies endeavoured to define the best empirical treatment, leading to specific guidelines (2,5-10). Broad-spectrum antibiotics are frequently prolonged until the symptoms and neutropenia subside. This long-term exposure can trigger side effects like allergies, renal or hepatic toxicity, and various forms of resistance due to selective antimicrobial pressure. The latest 4th European Conference on Infections in Leukaemia (ECIL-4) guidelines recommend to stop antibiotics in patients with Fever of Unknown Origin (FUO) after apyrexia for 48h or more, irrespective of their neutrophil count or expected durationof neutropenia (2). The possibility of earlier discontinuation of antibiotics, even if patients remain febrile, has rarely been studied (11-13). It is not recommended to stop antibiotics in patients with a clinical and/or microbiological documented infection due to excessive mortality.
Our primary objective was to study the feasibility and safety of a short-term antibiotic treatment in afebrile or febrile patients exhibiting FUO, irrespective of their neutrophil count. Our secondary objective was to describe the epidemiology of febrile neutropenia in our centre with clinical and biological criteria.
The primary objective of this study was to determine the feasibility and safety of a short-course antibiotic treatment for neutropenic patients exhibiting FUO. We demonstrate that discontinuation of empirical antibiotic therapy in FUO is safe in afebrile or febrile neutropenic patients. The latest ECIL-4 guidelines recommend discontinuing empirical antibiotics after at least 72h of intravenous administration in patients who have been haemodynamically stable since presentation and afebrile for 48h or more (2). In our experience, these recommendations are rarely applied and antibiotics continued until resolution of neutropenia. Persistent unexplained fever in neutropenia frequently appears to be non-infectious or non-bacterial (neoplastic fever, iatrogenic fever (chemotherapy, blood infusion, antibiotics), haemorrhage, thrombosis, viruses or fungal infections or febrile mucositis) (13,18-20).
From these observations, we hypothesized that antibiotics could be safely discontinued early, even if patients remain febrile and neutropenic. Three teams implemented early cessation of antibiotic therapy in patients with FUO (11-13). De Marie et al. stopped empirical antibiotic therapy at day3-5 for 25 haematology patients (11). Santoloya et al., in a randomized study among a population of children with cancer, implemented early cessation of antibiotic therapy for 36 patients (12). and Slobbe et al. terminated antibiotic therapy at day3 in 169 haematology patients at high risk of febrile neutropenia (13). No deaths or serious infections of bacterial cause were identified in these studies. In contrast to the studies of De Mary and Slobbe, we do not administer antibiotic prophylaxis to patients. Furthermore, our patients were carefully selected. Firstly, ECIL-4 recommendations were strictly applied then antibiotic therapy was stopped on day 5, irrespective of the temperature and the neutrophil count; comparison of the two groups of FUO revealed a significant reduction in the duration of antibiotic therapy without clinical complications, in accordance with previous studies .
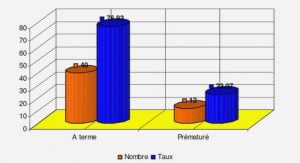
All patients had profound and prolonged neutropenia (leucocytes < 100/mm3) and were at high risk of severe febrile neutropenia due to their haematological malignancy and treatment (23). Our 2 groups differed in the type of chemotherapy and duration of neutropenia (24). This limit can be explained by the non-randomized approach and the small number of FUO patients. Of the patients to whom the study protocol was applied, most did not experience a recurrence of fever (60%), while 16.9% had another episode of FUO. For the other patients (23,1%), their CDI or MDI was immediately managed, without delay in the treatment of their haematological malignancy. As opposed to the report by Micol et al., we were able to stop antibiotic therapy as recommended by the ECIL. Seventeen patients did not receive the protocol due to poor adhesion of the medical and paramedical team, despite awareness of recommendations (medical and paramedical training courses). To eliminate inclusion bias, we checked the statistical analysis with 65 patients who received the study protocol. The results are the same as for the overall population of FUO (Data not shown). From the literature, with a reduced duration of antibiotherapy, patients are less exposed to selective antimicrobial pressure and its potential side effects (25). Our study cannot confirm this, because of the relatively short period of inclusion and the small number of patients with early cessation of antibiotic therapy
However, these encouraging results prompt us to continue with a larger, randomized study.
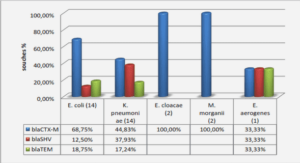
The secondary objective of our study was to analyse the clinical and biological characteristics of febrile neutropenia. In our population, we found a different repartition of FUO, MDI and CDI than in the literature (8,22,26-29) which may be explained by the use of more sensitive screening techniques (more selective middle culture, identification by mass spectrometry, better resolution CT) or different definitions of fever or MDI (8,22,26-30). All these items lead us to carefully compare our results with those of the literature. In our study, the most frequently identified bacteria were GNB (61,1%, mainly Escherichia coli) and GPC (37,6%, mainly CNS), as previously described (22,28,29,31-33). However, we noted a high level of piperacillin-tazobactam resistance in Escherichia coli isolates (27%).
This rate of resistance contrasts with the hospital data where E. coli resistant to piperacillintazobactam represent 6.5% (data from the years 2012, 2014 and 2015) and the departmental data from 2010 to 2012 where this resistance was measured at 15%. Mechanisms of resistance were diverse with penicillinase, cephalosporinase or extended spectrum betalactamase. Because of this contrast with hospital data and the use of this antibiotic for empirical antimicrobial therapy since 2013 in our department, this study highlights the need for local surveillance of microbial epidemiology in haematology centres, to allow the adjustment of empirical antibiotic therapy (34).
In this study, pneumoniae, neutropenic enterocolitis or mucositis were the most frequent sites of infection, which concurs with the literature (1,28-30). We considered neutropenic enterocolitis (from Nesher et al. (15)) and grade 3-4 mucositis as potential foci of infection, but sometimes without microbiological documentation. In the literature, the principle of « febrile mucositis » is reported (19). These mucosal lesions may cause a systemic inflammatory response syndrome, independently of a microbial infection. This mucosal damage is a known side effect of chemotherapy and can lead to secondary bacterial translocation, making it difficult to determine the border between chemotherapy-induced inflammation and infection (15,19,20). The overall in-hospital mortality rate was 10.5% (13/123 patients) and the infection related mortality was 7% (9/123 patients), more than in the literature (5-8% and 2-3% respectively) (22,27,29,30,32). These numbers may be explained by the profile of highly immunocompromised patients with an advanced chemotherapy program (salvage or transplant) and by severe coinfections. No deaths occurred precociously after antibiotic discontinuation.
|
Table des matières
REMERCIEMENTS
LISTE DES ABRÉVIATIONS
AVANT PROPOS
ARTICLE
KEY POINTS
ABSTRACT
INTRODUCTION
METHODS
RESULTS
DISCUSSION
REFERENCES
TABLE
Table 1 : Characteristics of included patients
Table 2 : Characteristic of patients with Fever of Unknown Origin
Table 3 : Distribution of pathogens isolated from bacteraemia and antibiotic susceptibility
Table 4 : Univariate analysis of factors associated with intensive care admission
FIGURE
Figure 1: Flow chart
SUPPLEMENTARY DATA
Figure 1: Outcome of FUO after application of the study protocol
Figure 2: Sites of infection for CDI patients
ANNEXES
ANNEXE 1 : AVIS DU COMITÉ D’ÉTHIQUE
ANNEXE 2 : DÉCLARATION NORMALE DE LA COMMISSION NATIONALE DE L’INFORMATIQUE ET DES LIBERTÉS
ANNEXE 3 : LETTRE D’INFORMATION AU PATIENT
ANNEXE 4 : PROTOCOLE DE SOINS UTILISÉ DU 01/02/14 AU 30/11/14
ANNEXE 5 : PROTOCOLE DE SOINS UTILISÉ DU 01/12/14 AU 30/09/15
ANNEXE 6 : DÉTAILS DES CAUSES DE TRANSFERT EN RÉANIMATION
ANNEXE 7 : DÉTAILS DES CAUSES DE DÉCÈS
ANNEXE 8 : TOP 10 DE L’ÉCOLOGIE BACTÉRIENNE DU SERVICE (HORS COLONISATION DIGESTIVE)
ANNEXE 9 : DÉTAILS DES RÉSISTANCES ANTIBIOTIQUES DES BACTÉRIES D’INTÉRÊT CLINIQUE
ANNEXE 10 : PROPOSITION DE NOUVEAU PROTOCOLE DE PRISE EN CHARGE DES NEUTROPÉNIES FÉBRILES
ANNEXE 11 : POSTER PRÉSENTÉ AU CONGRÈS DE LA SOCIÉTÉ FRANÇAISE D’HÉMATOLOGIE 2016
![]() Télécharger le rapport complet
Télécharger le rapport complet